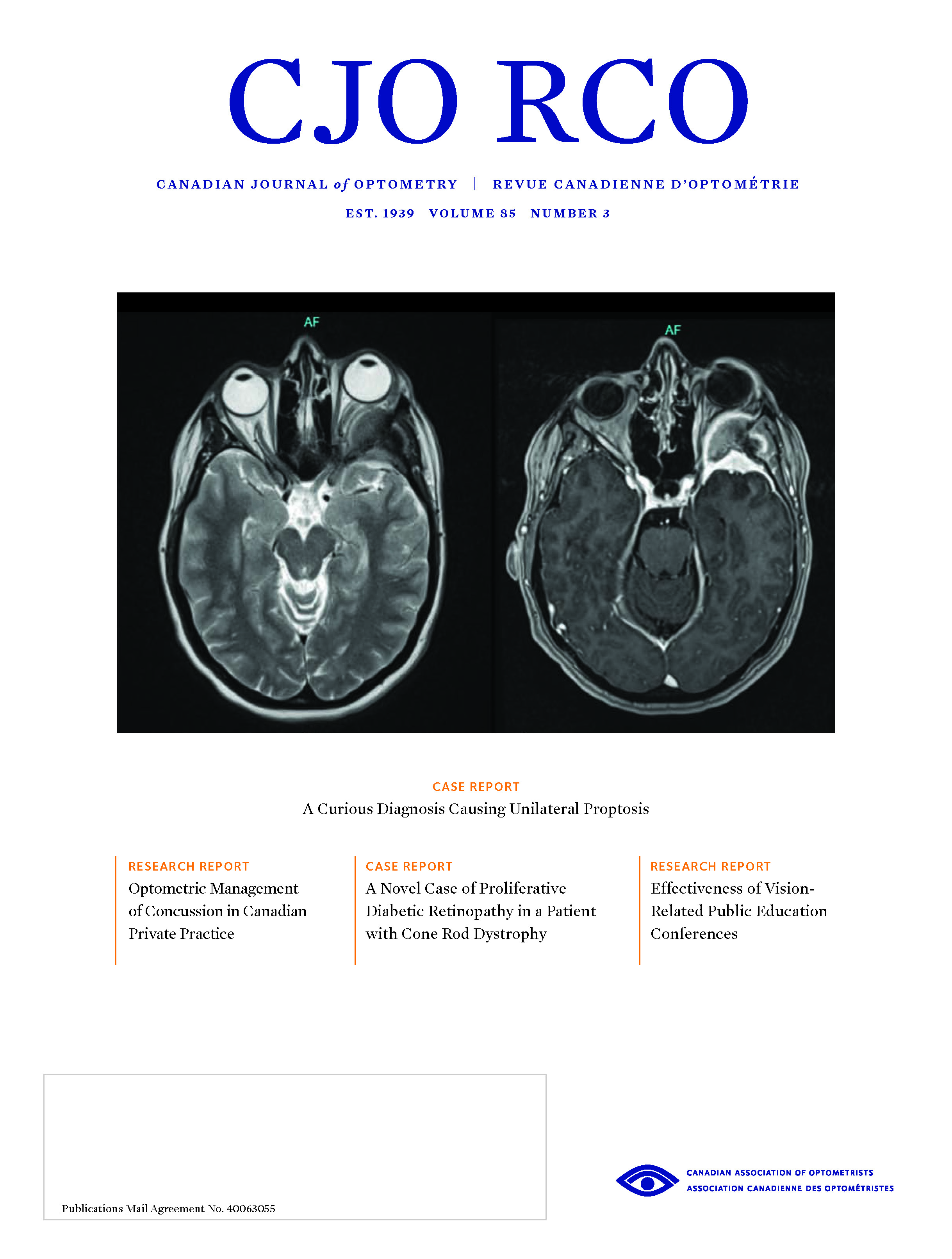
A Novel Case of Proliferative Diabetic Retinopathy In A Patient With Cone Rod Dystrophy
Abstract
Diabetic retinopathy and retinal dystrophies rarely present concurrently due to the protective effect of retinal dystrophies against diabetic retinopathy, and there is little written in the literature documenting the co-existence of diabetic retinopathy and retinal dystrophies. This report describes a rare case of proliferative diabetic retinopathy (PDR) in a patient with cone rod dystrophy (CRD). This co-existence is not only unique in its presentation but also highlights the important role of ancillary testing including fundus autofluorescence, fluorescein angiography, optical coherence tomography, and full field electroretinography. In addition to these, genetic testing also aids in the determination of the diagnosis. The pathophysiology of the concurrent development of these conditions is discussed and underlines the need for further investigation into the pathophysiology of diabetic retinopathy, particularly in patients with retinal dystrophies.
References
Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35(3):556-564.
Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis 2007;2:7.
Sternberg P, Landers MB, Wolbarsht M. The negative coincidence of retinitis pigmentosa and proliferative diabetic retinopathy. Am J Ophthalmol 1984;97(6):788-789.
Arden GB. The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. Br J Ophthalmol 2001;85(3):366-370.
Chen YF, Chen HY, Lin CC, Chen MS, Chen PC, Wang IJ. Retinitis pigmentosa reduces the risk of proliferative diabetic retinopathy: a nationwide population-based cohort study. PLoS ONE 7(9):e45189.
Preethi S, Rajalakshmi AR. Proliferative diabetic retinopathy in typical retinitis pigmentosa. BMJ Case Rep 2015;bcr2014-208589.
Bedoni N, Haer-Wigman L, Vaclavik V, et al. Mutations in the polyglutamylase gene TTLL5, expressed in photoreceptor cells and spermatozoa, are associated with cone-rod degeneration and reduced male fertility. Hum Mol Genet 2016;25(20):4546-4555.
Sergouniotis PI, Chakarova C, Murphy C, et al. Biallelic variants in TTLL5, encoding a tubulin glutamylase, cause retinal dystrophy. Am J Hum Genet 2014;94(5):760-769.
Dias MS, Hamel CP, Meunier I, et al. Novel splice-site mutation in TTLL5 causes cone dystrophy in a consanguineous family. Mol Vis 2017;23:131-139.
Krill AE, Deutman AF, Fishman M. The cone degenerations. Doc Ophthalmol 1973;35(1):1–80.
Sakuramoto H, Kuniyoshi K, Tsunoda K, Akahori M, Iwata T, Shimomura Y. Two siblings with late-onset cone-rod dystrophy and no visible macular degeneration. Clin Ophthalmol 2013;7:1703-1711.
Bax NM, Valkenburg D, Lambertus S, et al. Foveal sparing in central retinal dystrophies. Invest Ophthalmol Vis Sci 2019;60(10):3456-3467.
Sun X, Park JH, Gumerson J, et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci USA 2016;113(21):E2925-E2934.
Gill JS, Georgiou M, Kalitzeos A, Moore AT, Michaelides M. Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy. Br J Ophthalmol 2019;103(5):711-720.
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Keying Yan, Tybee Eleff, Zimei Zhou

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.